Anthropogenic CO2, the real %
Michel Thizon, a retired CNAM Paris engineer and former researcher at Ecole Polytechnique, has written a reaction to the research by Professor Demetris Koutsoyiannis, who argues that rising CO₂ levels are not the cause of global warming but its consequence (see for instance this article and YouTube video). Thizon writes: “Based on the measured 13C levels of atmospheric CO2, some have hypothesized that anthropic CO2 from fossil fuel combustion only represents 4 to 6% of atmospheric CO2. They thus conclude that human intervention is minor in the increase in atmospheric CO2. This is wrong for several reasons.”
We know of 15 isotopes of carbon, some of which are artificial and have very short lifetimes.
There are three carbons in nature.
-12C 98.93%, stable, 6 protons, 6 neutrons.
-13C 1.07%, stable, 6 protons, 7 neutrons.
-14C radioactive traces, half-life 5730 years, by cosmic radiation and nuclear activities.
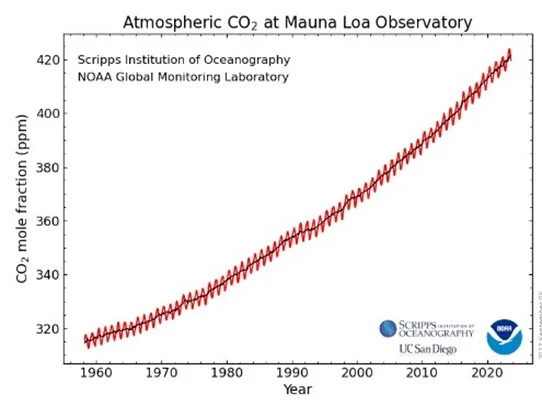
Plants absorb relatively less 13C than 12C. Thus, fossil fuels from the plant world (natural gas, oil, coal) have less 13C than in nature (carbonate rocks for example). Their combustion produces CO2 that is relatively depleted in 13C. Based on the measured 13C levels of atmospheric CO2, some have hypothesized that anthropic CO2 from fossil fuel combustion only represents 4 to 6% of atmospheric CO2. They thus conclude that human intervention is minor in the increase in atmospheric CO2, which has gone from 280 ppmv to 420 ppmv in 150 years. To justify the indisputable increase in atmospheric CO2 levels measured at the Mauna Loa Observatory (Hawaii), they imagine, for example, degassing of tropical oceans following a rise in ocean temperature that is only near 1°C.
This approach is wrong for several reasons.
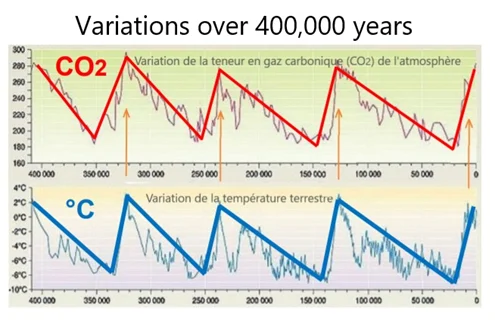
Firstly, analyses of Vostok ice cores (Antarctica) demonstrate, over eight regular cycles spread over 800,000 years, that a temperature variation of 1°C leads to an increase in natural CO2 of only about 8 or 10 ppmv. Even with a migration over several centuries, or even thousands of years, of gases captured in bubbles within the ice (an argument sometimes put forward to minimize these results), this does not change this conclusion.
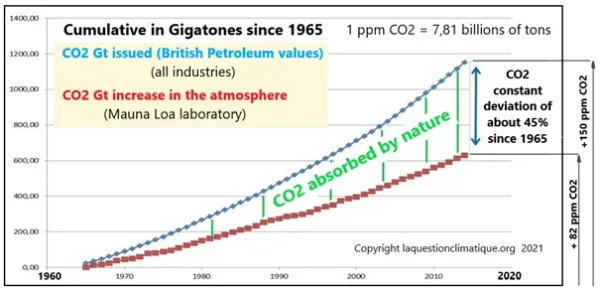
Secondly, global CO2 production is very precisely calculated by British Petroleum and other organizations (EDGAR, Carbon Global Project) from annual consumption of fossil fuels. Between 1965 and 2015 (50 years) 1150 gigatons of CO2 were emitted which would correspond to an additional 147 ppmv emitted into the air. In the same period Mauna Loa recorded an increase of 75 ppmv in the air because about half of the additional CO2 disappears, absorbed by nature (oceans and vegetation).
It can certainly be noted that carbon emissions and fluxes in nature are not limited to CO2 from human combustion, but the ‘baseline’ of CO2 measured was at a natural equilibrium level before this human intervention and corresponds to a constant.
The residual anthropic CO2 represents about 30% to 33% of the total CO2 in the air. If it were to be only 4 to 6%, more than 1000 gigatons, around 25% of the CO2 should have simply disappeared.
And thirldy, the CO2 molecules 12C and 13C are exchanged at the surface with the CO2 of the oceans. The oceans which cover 70% of the Earth’s surface and which contain, in different forms, tens of times more CO2 than the atmosphere, impose in the long run their 12C/13C ratio in the natural proportion. The kinetics of exchange are unknown and not studied (waves, winds, breaking waves, rains). Henry’s law, which regulates the ratio between gas content in water and in air in contact with the ocean surface, is respected. A significant exchange flow and regulation of the composition also exists between the CO2 degassed from warm tropical waters and the CO2 absorbed identically by cold waters.
(See: https://books.openedition.org/editionscnrs/11361)
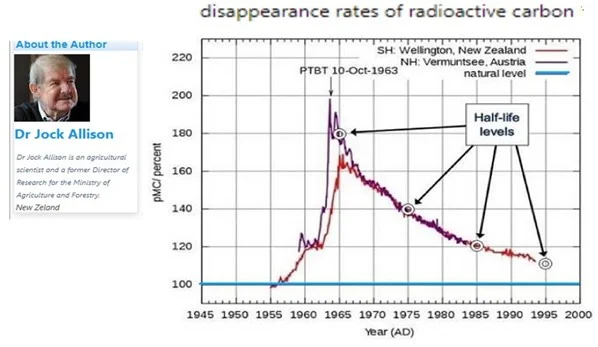
The proof of intense molecular exchanges at the water/air interface is provided by the progressive disappearance of radioactive CO2: 14CO2 from nuclear tests near 1960. Atmospheric CO2 with carbon 14 has a half-life of 10 years in the atmosphere, but its radioactive half-life is 5730 years. It therefore disappears, and relatively quickly, by molecular exchanges with oceanic CO2 at the ocean/atmosphere interface.
(See: https://www.nzcpr.com/carbon-dioxide-is-a-short-lived-gas/#more-27750)
Ultimately, in a relatively few years, about 50% of the additional CO2 disappears into nature. If we consider the case of 14C, we can imagine that it is of the order of magnitude of ten years, and not 1000 years or even 100 years as some qualified ‘climatologists’ attached to the IPCC, claim without scientific approach. The oceans do not have an infinite capacity for exchange and absorption, but we must not forget the oceanic phenomena, also terrestrial, which consist in continuously transforming CO2 into calcium minerals.
Conclusion
Although, strictly speaking, the residual CO2 from fossil fuels only represents 4 to 6% after molecular exchanges at the water/air interface, the 50% increase in global atmospheric CO2 over the past 150 years is indeed due to combustion, i.e. a current CO2 content caused by anthropic activities of approximately 1/3. But we are entitled to think that if we slowed down emissions significantly, or even if we remained at a stable level for several years, atmospheric CO2 ppm would decrease in a few years without it being necessary to fall to zero emissions as claimed by the alarmists. On the other hand, the atmospheric CO2 content had in the past several times reached a critical lower limit for plant growth and nowadays a higher content than then can only be favorable to them.
more news
Clearing up some misconceptions about the DoE report
After environmental groups sued to halt a critical Department of Energy climate report, accusations of secrecy and political bias quickly followed. Ross McKitrick responds by challenging what he calls widespread misconceptions about the project, defending its transparency, scientific grounding and editorial independence.
India Builds a ‘Fossil Future’
While Western governments continue to speak the language of net zero, India is rapidly expanding coal, oil and natural gas production to secure long-term energy security and economic growth. By strengthening hydrocarbon trade with the United States and other partners, India is building what the author calls a “fossil future,” prioritizing reliable and affordable energy over climate pledges.
Interview with Dr. Guus Berkhout: A Different Perspective on Climate Science and Energy Policy
The big problem today is that climate models are not fit-for-purpose, says Clintel co-founder dr. Guus Berkhout. They do not reflect the real world. That is the reason why the Net Zero policy does not work. We need fundamental changes in climate science and climate policies. We now see that this message gets more and more support.